Biosimilars - the Art of Designing a Clever Path

Whereas in the development of NBE (new biological entities) the focus is on proving safety and efficacy by extensive preclinical and clinical phase I, II and III testing, the focus in biosimilar development is on the quality aspects: what is the degree of chemical and biological similarity of my biosimilar with the originator.
Cell line selection and manufacturing process development and optimization with back and forth test laboratory involvement will help to end up with a highly similar candidate that reduces the extent of (non-) clinical studies.
We will help you to rationalize your science-based biosimilar package based on regulatory demands combined with your in-house knowledge on cell line, process and product.
Analytical, Functional
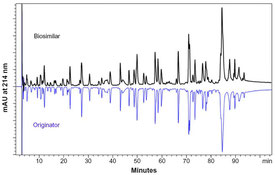
As the pilar for further development of your biosimilar candidate, extensive analytical and functional characterization and comparability testing is essential. We help you to select a comprehensive and tailor-made test package that enables you to show biosimilarity and reduce (non-) clinical package.
Non-Clinical

The relevance and completeness of your analytical and functional package determines the amount of preclinical testing.
The degree of certainty the data provide determine the need for pre-clinical studies, if any.
Clinical

Fast but tailor-made and cost-effective clinical trials may significantly contribute to the speed of market introduction of your candidate.
We have an extensive network of science based clinical and bioanalytical providers that provide speed and good pricing.
Antaeus Biopharma Consulting & Services - Verlaat 1 - 8428RS FOCHTELOO - The Netherlands
Phone: +31 6 2033 5602 - info@antaeus-biopharma.com